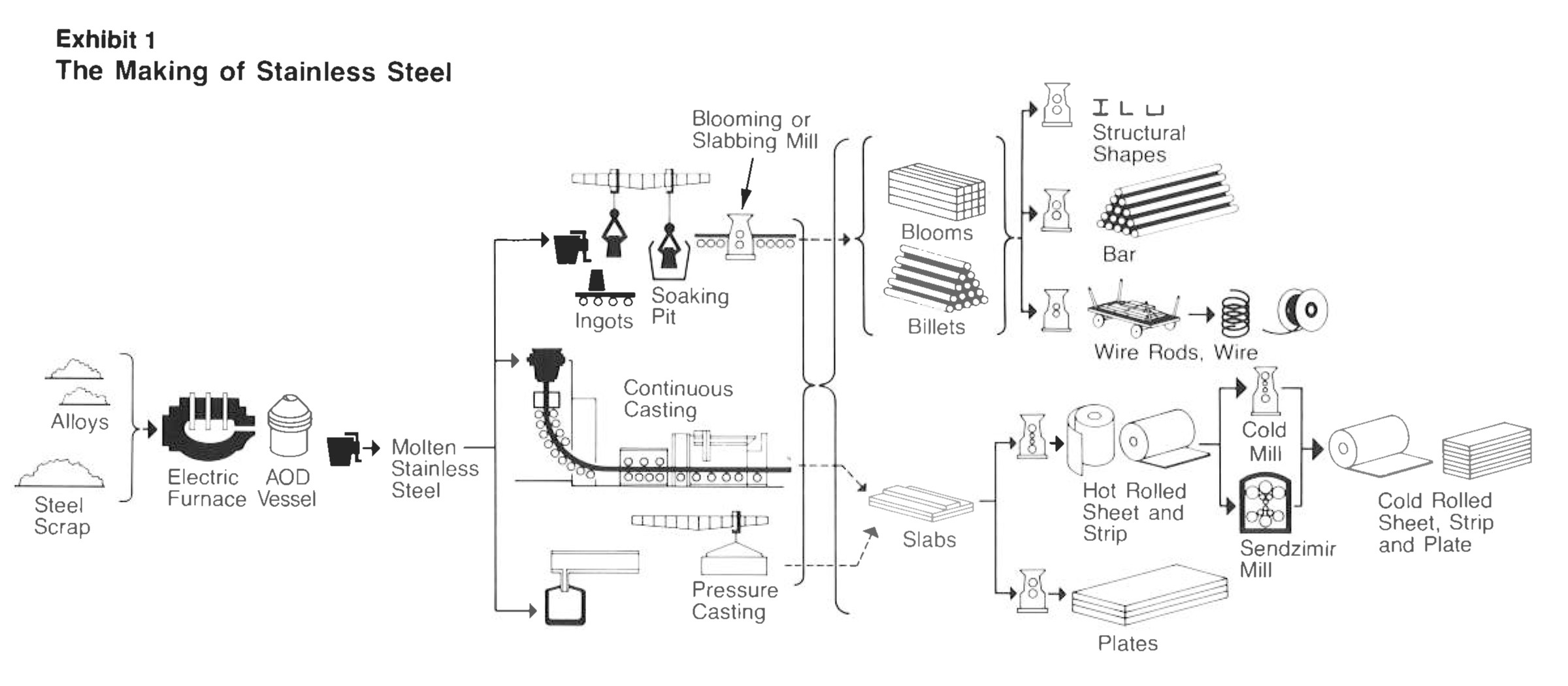
How is Stainless Steel Made?
Stainless steel is produced in an electric furnace where carbon electrodes contact recycled stainless steel scrap and various alloys of chromium ( and nickel, molybdenum etc.depending on the stainless steel type). Current is passed through the electrode and the temperature increase to a point where the scrap and alloys melt.
The molten material from the electric furnace is then transferred into and AOD( Argon Oxygen Decarborization) vessel , where the carbon levels are reduced( remember stainless has a much lower carbon level than mild steel) and the final alloy additions are made to achieve the exact chemistry.
Exhibit 1 shows the process, which starts with melting and casting into the stainless steel into either ingots or continuous casting into a slab or billet form. Then the material is hot rolled or forged into its final form. Some material receives cold rolling to further reduce the thickness to create sheet or strip or it is drawn into smaller diameters to make rod and wire.
Most stainless steel receive a final annealing( a heat treatment that softens the structure) and pickling( an acid wash that removes the furnace scale from annealing and helps promote the passive surface film that naturally occurs).